
Our client needed to assess the safety profile of a bispecific T cell engager. While they had gathered some information from cell lines and animal studies, they needed more accurate, human-relevant data to better understand the tumor-associated antigen (TAA) expression in real patient tissues and its potential effects in normal non-cancerous cells. This was crucial for assessing the safety and efficacy of their bispecific T cell engager in a more clinically predictive context.

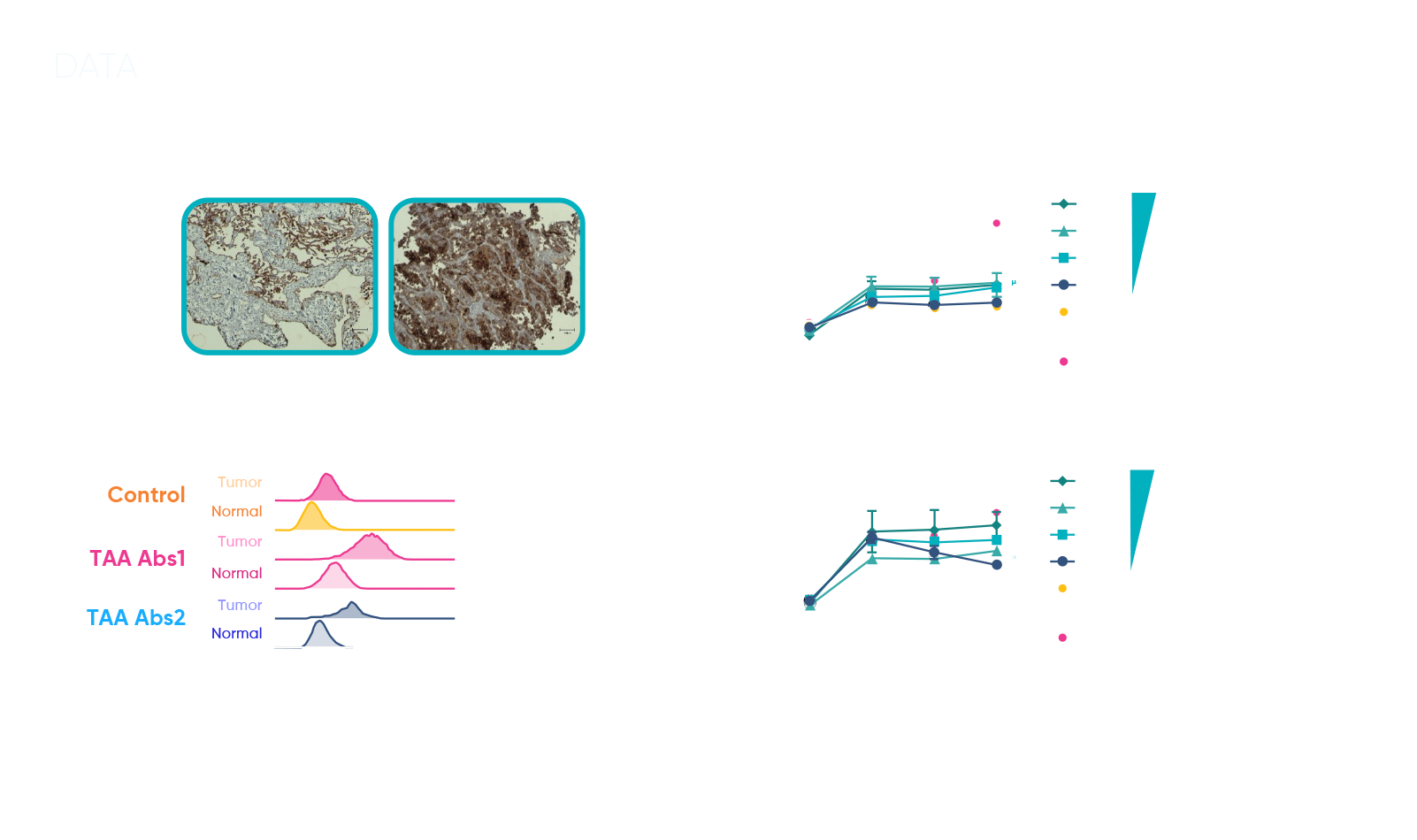
To provide a more clinically predictive safety profile, we utilized organoid and T cell co-cultures derived from both normal and tumor tissues from the same patient. This approach allowed us to simulate a human-relevant environment, offering a clear comparison of how the bispecific T cell engager impacted both cancerous and non-cancerous cells. By examining tumor-associated antigen (TAA) expression across matched healthy and diseased tissues, we were able to pinpoint on-target activity while assessing any potential off-target toxicity.

The sample data demonstrated distinct interactions between the bispecific T cell engager and TAA expression in normal versus tumor-derived organoid co-cultures. The results provided critical insights into how the compound engaged T cells in tumor tissues while minimizing effects on healthy cells. Armed with this patient-relevant data, the client was able to refine the therapeutic window, moving forward with a clearer understanding of the compound’s safety and efficacy profile.