
The client approached us to assess the GI toxicity of an anticancer agent before progressing to animal studies. With no prior data on the agent’s gastrointestinal effects, they sought to identify the most human-relevant animal model, ensuring that their choice would support accurate translation to human clinical trials.

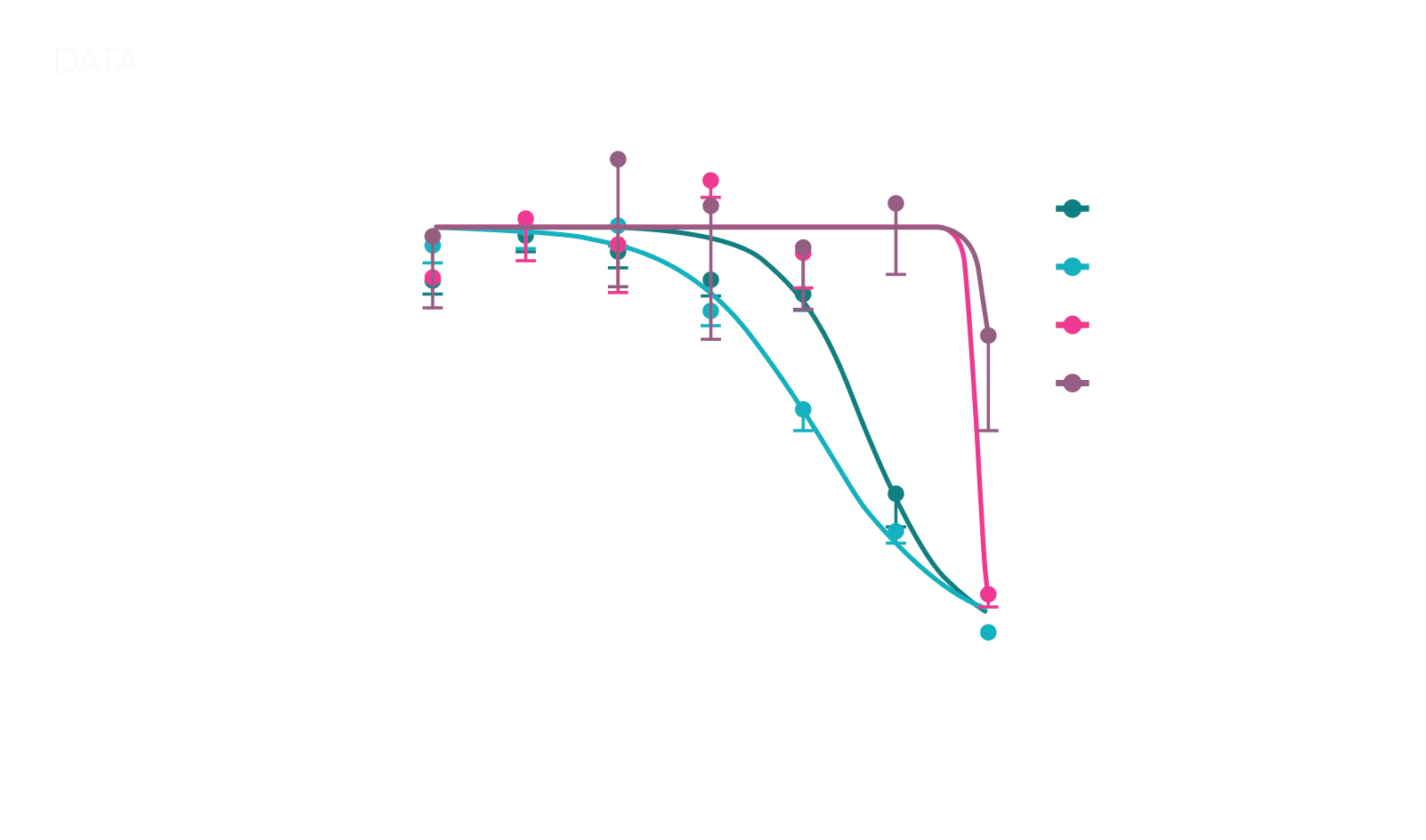
After an in-depth literature review, we selected human, dog, rat, and mini-pig as the comparative models for an in vitro toxicity screen. From our extensive biobank, we sourced well-characterized small intestinal and colon organoids. To best fit the client’s compound, we customized the screen design, refining both treatment windows and readouts, and developed an ATP-based viability assay to deliver reliable and specific toxicity data.

Our testing revealed that dog organoids exhibited toxicity profiles most similar to human organoids, while mini-pig and rat profiles diverged significantly. This compelling evidence enabled the client to select dogs as their primary non-rodent model, confidently advancing their in vivo studies with a high likelihood of predictive, human-relevant results.