
Whereas the CRISPR/Cas9 technology cuts out a defect in a gene and replaces it with a new piece, the latest CRISPR technology works differently. The goal is to repair the error in the DNA without cutting it. Scientists from the research groups of Hans Clevers (Hubrecht Institute) and Jeffrey Beekman (UMC Utrecht) show for the first time that this technique can effectively and safely repair the DNA of organoids derived from cystic fibrosis patients in the lab.
In 2018 a new CRISPR-enzyme was developed that makes the CRISPR technique more precise and less error-prone, according to biologists Maarten Geurts (Hubrecht Institute) and Eyleen de Poel (UMC Utrecht). Maarten: “In traditional CRISPR/Cas9 genome editing a specific piece of the DNA is cut out resulting in DNA damage. This is done with the aim that the cell repairs this cut using a lab-made piece of ‘healthy’ DNA. However, in the new CRISPR-technique, called base editing, the Cas-part is altered in such a way that it no longer creates a cut, but still detects the mutation. So, instead of creating a cut and replacing the faulty DNA, the mutation is directly repaired on site, making this a more effective genome editing tool.”
Organoids
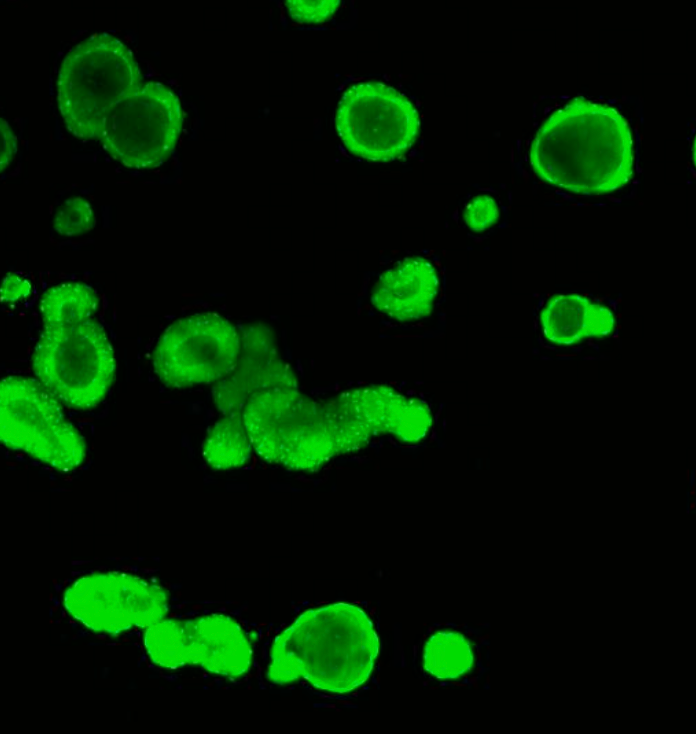
Hubrecht Organoid Technology and UMC Utrecht have generated a living biobank consisting of intestinal organoids derived from 664 patients with Cystic Fibrosis (CF). At Hubrecht Organoid Technology, HUB Organoids are used to determine which medicine works for which CF patient, but also to test new treatments, such as CRISPR/Cas. In this study, organoids were used to investigate whether CRISPR base-editing works on the DNA in human adult stem cells, and whether it can be used to cure CF in the `mini-intestines`.
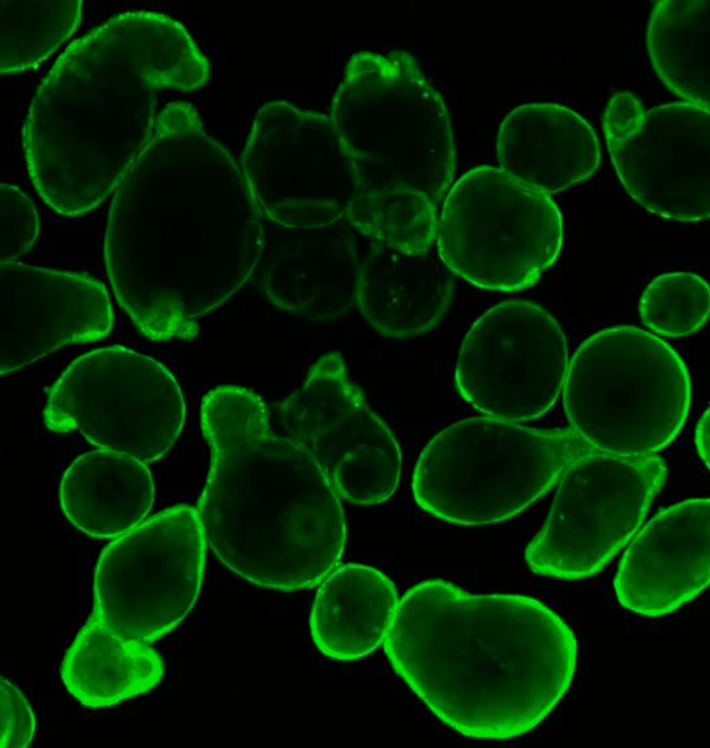
Challenges in CF
CF is a genetic disease that is caused by mutations of the gene encoding for the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Mutations in CFTR gen lead to impaired protein function causing severe damage to the lungs, digestive system and other organs in the body. A major problem in treating CF is the diversity of the genetic defect. More than 2000 different mutations have been identified in CF patients, with only 12 common mutations being represented in 50% of the CF population and more than 1900 rare mutations being distributed in the other 50%. For CF patients with common mutations, effective medication is available. However, for the group with rare mutations, there is no treatment available emphasizing the importance and the value of patient-derived organoids in drug development and diagnostic.
Publication
CRISPR-based adenine editors correct nonsense mutations in a cystic fibrosis organoid biobank. M.H. Geurts*, E. de Poel*, G.D. Amatngalim, R. Oka, F.M. Meijers, E. Kruisselbrink, P. van Mourik, G. Berkers, K.M. de Winter-de Groot, S. Michel, D. Muilwijk, B.L. Aalbers, J. Mullenders, S.F. Boj, S.W.F. Suen3, J.E. Brunsveld, H.M. Janssens, M.A. Mall, S.Y. Graeber, R. van Boxtel, C.K. van der Ent, J.M. Beekman†, H. Clevers†. Cell Stem Cell 2020.




