POSTER PRESENTATION
Directly derived from patient tissue and never passaged in host animals
Test off-tumor toxicities of your ADCs in matched normal organoids
Recapitulates the structure, function, and cellular profiles of patient tumor
Reflects variability observed in patient response, despite similar antigen expression
THE CHALLENGE
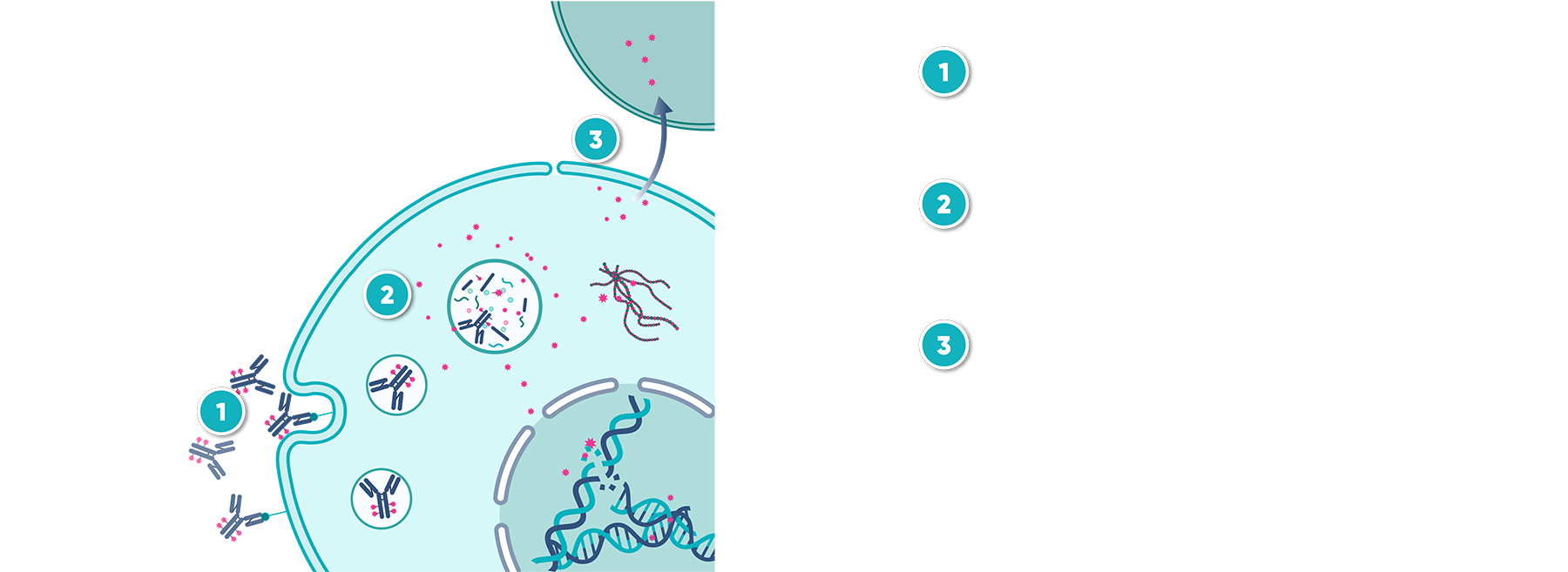
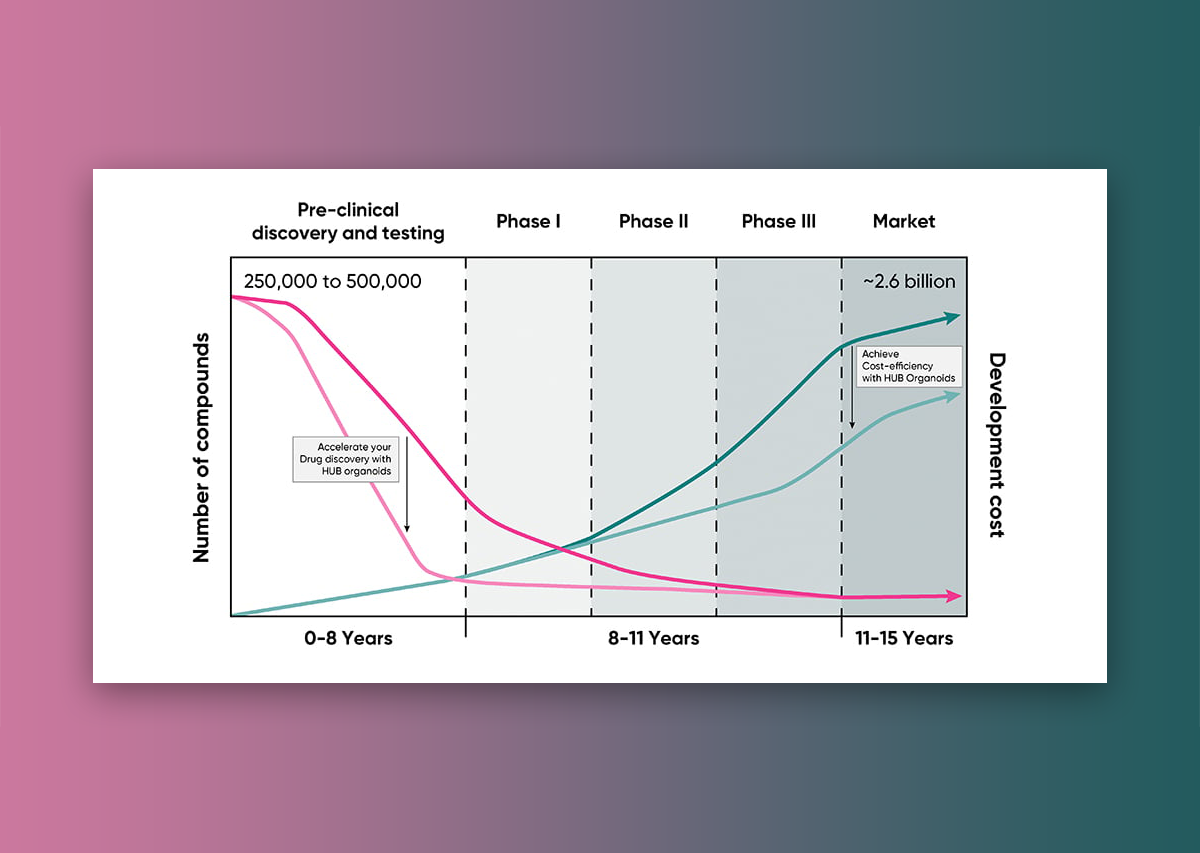
Antibody-drug conjugates (ADCs) have achieved significant milestones, with 15 FDA approvals since 2000, due to their targeted mechanism of action. However, the drug development process remains highly complex, largely due to intricate pharmacodynamics and the limitations of current experimental systems. For instance, cell lines often overexpress target antigens, which can result in efficacy assessments that do not accurately reflect clinical conditions. Although patient-derived xenograft (PDX) models offer a more accurate representation of human tumors, they are expensive and may not capture the full spectrum of tumor variations. Furthermore, animal models often fall short in mimicking the complexity of human tumor biology and immune responses, with species differences further complicating the translation of preclinical findings to clinical outcomes.
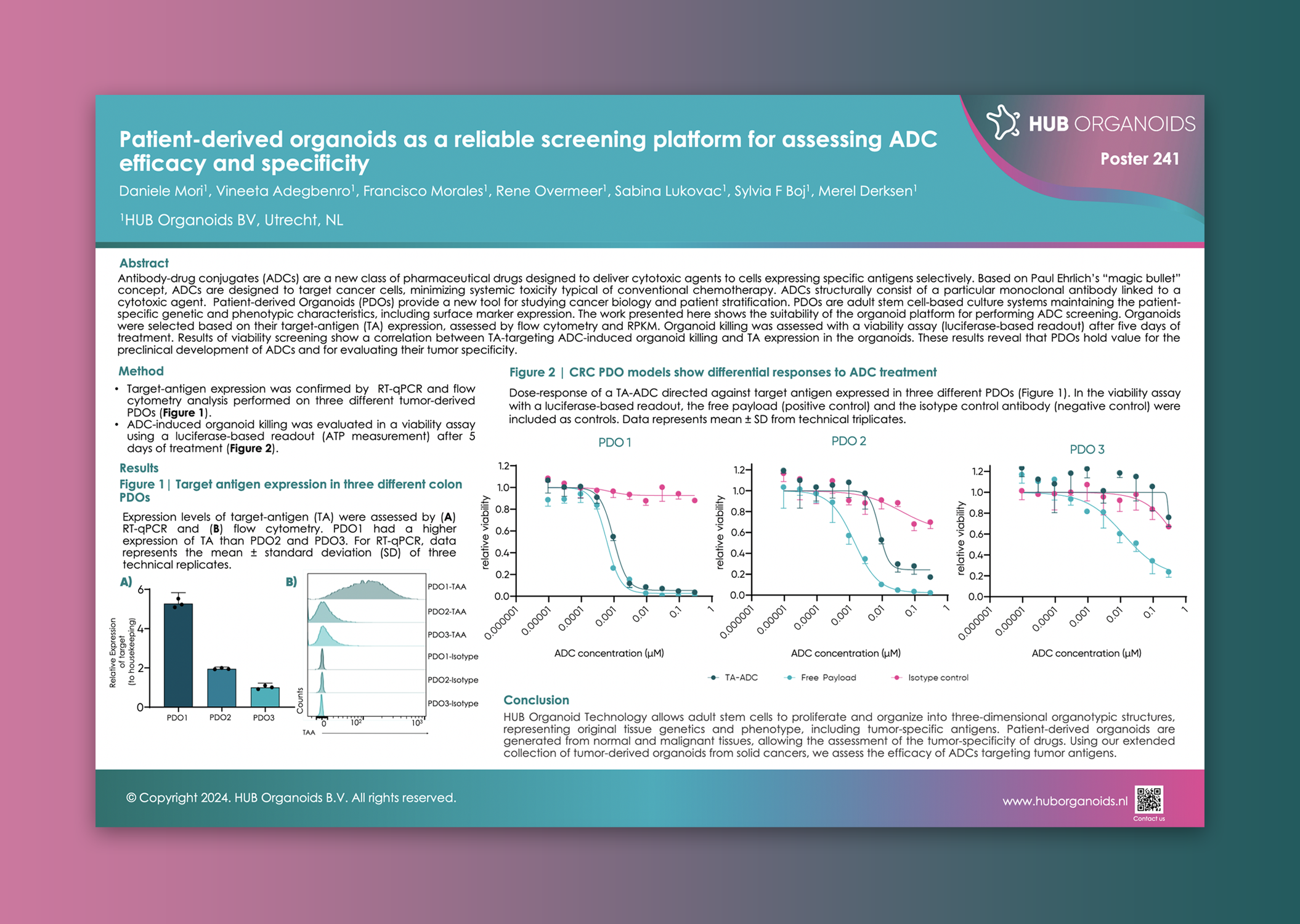
| OrganoID-Flow Target Antigen Expression Analysis |
ADC Efficacy Screen Potency Assessment Across Tumor Types |
Unique solutionADC Optimization Screen Comprehensive Efficacy and Safety Profiling |
|
|---|---|---|---|
| Ideal for | Informed selection of cancer indications based on precise TAA profiling | Determine the efficacy and potency of your ADC candidates across a diverse panel of tumor organoids. | Simultaneously evaluate ADC efficacy and potential off-target/off-tumor effects |
| Features | Accurate assessment of TAA expression across various patient-derived organoids via flow cytometry. |
|
|
POSTER PRESENTATION
BLOG