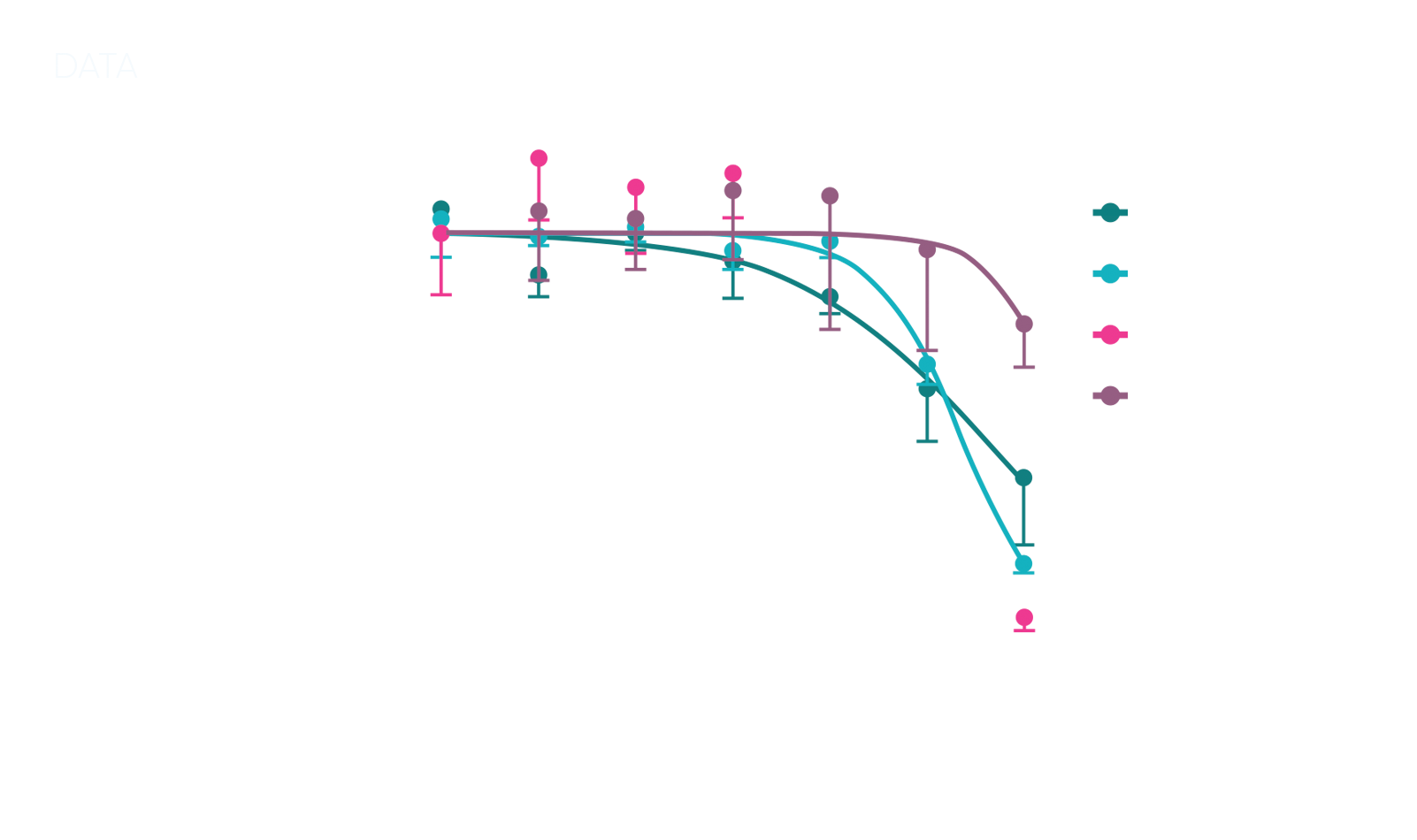


Our client’s in vivo testing results showed gastrointestinal (GI) toxicity in non-rodent species dogs, but not a similar profile in rats, making it unclear if the toxicity would be relevant to human outcomes in future trials. To progress, they needed to determine whether this toxicity profile in dogs would translate to humans.

To clarify the compound’s toxicity potential, we conducted an in vitro screening using organoids derived from human, dog, mini-pig, and rat tissues. Each organoid model was carefully selected to match the client’s needs for relevant GI toxicity markers, and our team designed a screen that allowed precise comparisons of toxicity profiles across species.

The data showed that the dog organoids exhibited toxicity patterns that closely matched those of human organoids, while the rat organoids did not align with human results. Armed with this insight, our client identified a potential therapeutic window and gained confidence that their drug’s GI toxicity in dogs would likely translate to humans, guiding them in refining their preclinical strategy